Health
World Marks 37th World AIDS Day as Funding Cuts Raise Fears of Setbacks in Global Fight Against HIV

Health
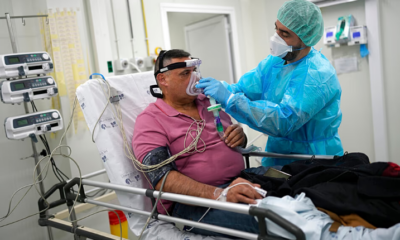
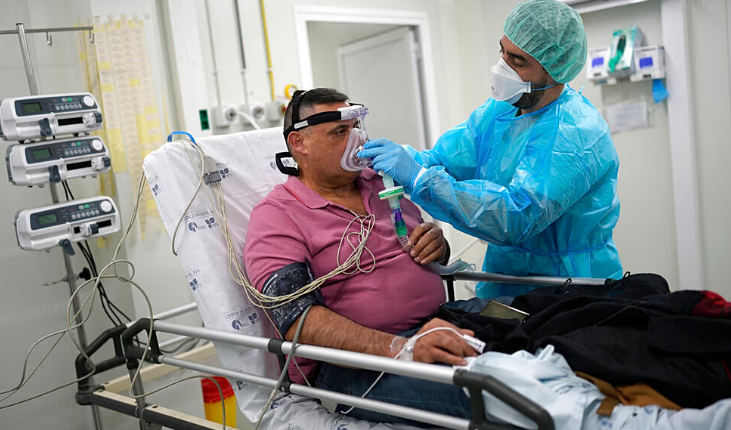
Early Flu Wave Hits Europe as Experts Offer Tips to Ease Symptoms

Most people catch a cold in winter, battling fatigue, runny noses, sneezes, coughs, and congestion. This season, an unusually early flu wave has affected millions across Europe, prompting health authorities to offer guidance on managing symptoms.
Typically, influenza season runs from mid-November to late May. However, figures from the European Centre for Disease Prevention and Control (ECDC) indicate that cases in 2025 surged three to four weeks earlier than in the previous two seasons. The agency reports that flu circulation remains high, though it has recently peaked in most regions.
While there are no specific cures for the flu and the common cold, some simple measures can help manage symptoms and improve comfort. Experts recommend a combination of hydration, rest, and symptom relief to support recovery.
Drinking enough fluids is particularly important. Water, broths, and warm beverages with honey, lemon, or ginger can help prevent dehydration, loosen mucus, and soothe irritated airways. Health authorities caution against alcohol, coffee, and other caffeinated drinks, which can act as diuretics and worsen congestion and sore throats.
Gargling with warm salt water is another simple measure to ease throat pain and reduce inflammation. Mixing one teaspoon of salt in a cup of warm water and using it several times a day can provide relief and help clear mucus.
Rest is essential for recovery. Adults are advised to sleep seven to nine hours a night, and to take naps as needed to allow the immune system to combat the virus. Light exercise such as short walks or yoga may be suitable for mild symptoms, but physical activity should be avoided if fever is present or chest symptoms worsen, to prevent complications.
Maintaining moisture in the environment can also alleviate symptoms. Dry air worsens congestion and sore throats, while humidified air hydrates airways and helps clear mucus. Higher humidity can also reduce how long some respiratory viruses survive in the air.
Medicines can relieve symptoms but do not cure viral infections. Decongestants, such as saline sprays or drops, can ease nasal congestion. Painkillers, including ibuprofen, may reduce fever and discomfort in adults. Antibiotics are not effective against colds or flu and should be avoided to prevent antibiotic resistance.
Health authorities emphasize that these measures, combined with good hygiene and vaccination where appropriate, are key to limiting the impact of seasonal viruses. The ECDC notes that most healthy adults recover from flu within about a week, while a common cold may last up to two weeks.
With the early flu wave affecting millions across Europe, simple steps such as staying hydrated, resting, and taking appropriate symptom relief can help patients recover safely and reduce strain on healthcare services.
Health
Climate Change Drives Respiratory Illness, Push for Low-Carbon Inhalers Grows
As climate change worsens respiratory diseases, doctors and drugmakers are exploring earlier diagnosis and low-carbon inhalers to cut emissions from care and protect patients.
For millions of people worldwide, climate change is already affecting breathing. Rising air pollution, longer pollen seasons, and smoke from wildfires are contributing to asthma attacks, lung damage, and other chronic respiratory illnesses. At the same time, the health systems treating these conditions are themselves contributing to greenhouse gas emissions.
Experts note that over 90 percent of the global population breathe air with particulate levels above World Health Organization recommendations. Climate extremes and poor air quality are increasing the frequency and severity of respiratory diseases, from exacerbations to disease onset. Therese Laperre, head of the respiratory department at University Hospital Antwerp, said changes in particulate matter can trigger emergency visits for asthma and chronic pulmonary diseases days later. A European Environment Agency study estimated that more than a third of chronic respiratory disease deaths in Europe are linked to environmental factors such as pollution, extreme temperatures, wildfire smoke, and allergenic pollen.
Worldwide, an estimated 400 to 500 million adults live with COPD, and over 250 million have asthma. Health care responses come with their own climate cost. Health Care Without Harm estimates global health services generate about five percent of worldwide greenhouse gas emissions, which could rise to six gigatons annually by 2050 if unchecked, equivalent to more than a billion cars on the road. Hospitals, particularly intensive care units, are among the most carbon-intensive, due to high energy use, equipment, and single-use materials.
Respiratory specialists emphasize that early disease control benefits both patients and the environment. Philippe Tieghem from the French respiratory association Sante Respiratoire said early diagnosis allows for better patient care while reducing emissions.
Inhalers, commonly used to treat asthma and COPD, illustrate the challenge. Traditional pressurised metered-dose inhalers (pMDIs) use hydrofluorocarbon propellants, which contribute 16–17 million tonnes of CO₂-equivalent emissions globally each year. The UK’s National Health Service estimates these inhalers account for around three percent of its carbon footprint.
Pharmaceutical companies are developing greener alternatives. AstraZeneca’s reformulated COPD inhaler uses a new propellant, HFO‑1234ze(E), cutting its global warming potential by roughly 99.9 percent compared with older devices. The company has pledged to reduce its emissions by 98 percent by 2026, starting with inhalers. Pfizer and Johnson & Johnson have committed to net-zero targets by 2040 and 2045, respectively.
Pablo Panella, senior vice-president for respiratory diseases at AstraZeneca, told Euronews Health that early detection, diagnosis, and treatment keep patients controlled in the community, reducing hospital admissions and the high-carbon interventions that come with them. Panella also stressed the importance of regulation that supports innovation, allowing low-carbon technologies to reach patients more quickly.
Better disease control, combined with environmentally conscious products, is creating the concept of the “green patient”: someone whose chronic condition is managed well enough to avoid repeated flare-ups, hospital stays, and carbon-intensive treatments.
Health
Experts Offer Tips to Beat Winter Blues on “Blue Monday”

Monday, 19 January, is often called Blue Monday, reportedly the “saddest day of the year.” The term was coined in 2005 by UK psychologist Cliff Arnall for a travel company, Sky Travel, as a way to promote winter holidays. Arnall’s formula combined weather data, debt levels, time since Christmas, motivation, and the status of New Year’s resolutions to identify the third Monday of January as particularly gloomy. However, experts say there is no scientific evidence supporting the idea that this day is inherently sadder than any other.
While Blue Monday itself is not backed by research, the winter months can be challenging for many people. Shorter daylight hours, colder temperatures, and reduced outdoor activity contribute to what is often referred to as the “winter blues.” Seasonal Affective Disorder (SAD), a type of depression linked to seasonal changes, is most common during late autumn and winter. Symptoms include low mood, fatigue, difficulty concentrating, disrupted sleep, and a loss of interest in usual activities. Experts link SAD to reduced sunlight, which can affect serotonin and melatonin, the body’s natural chemicals that regulate mood and energy.
Happiness expert Stephanie Davies says overcoming the winter slump does not require drastic changes, but small, intentional steps that support mental well-being. Regular exercise, exposure to sunlight, good sleep, and social connection can all help counter the seasonal dip in mood.
Exercise, even for 20 minutes a day, can release endorphins and improve motivation. Outdoor activity is particularly beneficial, as daylight exposure helps regulate sleep patterns and boost energy. Research has shown that physical activity can be as effective as therapy or medication in easing symptoms of depression, including fatigue and sadness.
Sunlight exposure is also critical. Natural light helps adjust the body’s internal clock and increases serotonin levels, improving energy and alertness. Experts recommend keeping living spaces bright and spending time outdoors when possible. Light therapy is another effective treatment for those suffering from SAD.
Maintaining a regular sleep schedule is equally important. Adults generally need seven to nine hours of sleep a night, and maintaining consistent sleep patterns helps regulate circadian rhythms. Excessive napping or irregular sleep can worsen low energy and mood.
Social connection provides another important benefit. Reduced daylight and colder weather often lead people to stay home, increasing feelings of isolation. Whether through face-to-face interaction, phone calls, or messages, maintaining contact with friends and family can help reduce loneliness and improve overall well-being.
While Blue Monday may be more of a media concept than a scientific fact, experts agree that proactive steps to maintain activity, light exposure, sleep, and social contact can make the winter months more manageable and support both mental and physical health.
-

 Entertainment1 year ago
Entertainment1 year agoMeta Acquires Tilda Swinton VR Doc ‘Impulse: Playing With Reality’
-

 Business2 years ago
Business2 years agoSaudi Arabia’s Model for Sustainable Aviation Practices
-

 Business2 years ago
Business2 years agoRecent Developments in Small Business Taxes
-

 Home Improvement1 year ago
Home Improvement1 year agoEffective Drain Cleaning: A Key to a Healthy Plumbing System
-

 Politics2 years ago
Politics2 years agoWho was Ebrahim Raisi and his status in Iranian Politics?
-

 Business2 years ago
Business2 years agoCarrectly: Revolutionizing Car Care in Chicago
-

 Sports1 year ago
Sports1 year agoKeely Hodgkinson Wins Britain’s First Athletics Gold at Paris Olympics in 800m
-

 Business2 years ago
Business2 years agoSaudi Arabia: Foreign Direct Investment Rises by 5.6% in Q1