Health
UK Lawmakers to Vote on Assisted Dying Legislation Amid European Context
LONDON — The United Kingdom is set to take a significant step in its debate over assisted dying as lawmakers prepare to vote on a proposed bill on Friday. If passed, the legislation would allow terminally ill adults in England and Wales to request medical assistance to end their lives, placing the UK among a small number of European nations with such laws.
The proposed bill outlines strict safeguards to prevent abuse, including the requirement for individuals to have the mental capacity to make the decision, provide two formal declarations of their intent, undergo evaluations by two doctors within a week, and receive approval from a high court judge. Eligibility would be limited to adults with a life expectancy of six months or less, who would self-administer an approved substance to end their lives.
This draft legislation has reignited discussions about the ethical, legal, and societal implications of assisted dying in the UK. Proponents argue for the right to choose how and when to die, while critics emphasize the need to protect vulnerable individuals from coercion.
Assisted Dying in Europe
Across Europe, only a handful of countries permit euthanasia or assisted suicide, with many implementing such laws relatively recently.
The Netherlands was the first nation globally to legalize euthanasia in 2002, under strict conditions ensuring that the patient’s request is voluntary and well-informed, their suffering is unbearable with no prospect of improvement, and all alternatives have been exhausted. Minors aged 12 and above may also request euthanasia with parental consent.
Belgium followed shortly after, adopting a similar law in 2002, which it later extended to minors in 2014. Luxembourg decriminalized euthanasia and assisted suicide in 2009, and Spain introduced legislation in 2021 for individuals experiencing unbearable suffering.
In Portugal, a long-debated euthanasia bill was passed in 2023 despite initial vetoes by the president. Meanwhile, Germany and Austria changed their laws following constitutional rulings that upheld the right to self-determination in end-of-life decisions.
Ongoing Debates
While some European nations have embraced assisted dying, others remain divided. Ireland’s parliament is considering recommendations for legalizing assisted dying, while France is set to debate comprehensive legislation in 2025.
In the UK, where assisted suicide and euthanasia are currently illegal, this legislative push represents a critical moment in a decades-long debate. Advocates and critics alike are watching closely as lawmakers decide whether the UK will join its European counterparts in legalizing the practice under controlled circumstances.
The vote is expected to draw widespread attention, reflecting the growing global conversation about autonomy, dignity, and the ethics of end-of-life care.
Health
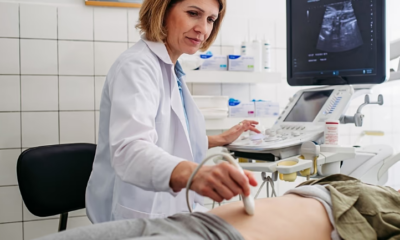
FDA Clears AI Tool to Improve Detection of Fetal Abnormalities in Ultrasounds
A new artificial intelligence software designed to enhance prenatal ultrasound screenings has received clearance from the United States Food and Drug Administration (FDA), offering a potential boost to the detection of fetal abnormalities.
Developed by the American start-up BioticsAI, the tool integrates with existing ultrasound machines to analyze images in real time, highlighting potential issues during scans. Prenatal ultrasounds are widely used throughout pregnancy to identify potential problems in a developing fetus, including malformations in organs or limbs. Yet studies suggest that routine scans can miss a significant number of abnormalities.
According to research, a single early scan performed between 11 and 14 weeks of pregnancy detects only about 38 percent of birth defects. A mid-pregnancy scan, typically conducted between 18 and 24 weeks, identifies roughly 51 percent of abnormalities. When both scans are performed, detection rises to 84 percent, leaving a remaining gap in diagnosis.
BioticsAI’s software works by analyzing each fetal image as it is captured. It evaluates image quality, suggesting adjustments to ensure a clear view of the fetus, and checks whether all parts of the baby are visible. Using data patterns drawn from a global database, the system can detect anomalies, including heart or limb defects, and flag them for the doctor during the scan. After the examination, the software generates a report compiling all findings for clinical review.
Developers say the tool can also save healthcare professionals roughly eight minutes per patient in documentation time. The FDA’s clearance confirms that the software meets medical device performance standards and can be safely integrated into current ultrasound systems.
The approval comes amid ongoing challenges in prenatal care. In Europe, major congenital anomalies occur in about 23.9 per 10,000 births. AI-driven tools are emerging as a promising supplement to conventional scans. French companies Diagnoly and Sonio Detect have also received approval for AI-assisted ultrasound solutions, which automatically identify fetal structures and detect potential heart issues.
Experts say integrating AI into prenatal care could improve early detection rates and help doctors provide timely interventions or monitoring. Real-time feedback during scans ensures that images are complete and abnormalities are less likely to be missed.
BioticsAI’s FDA-cleared tool is expected to be rolled out in clinics across the United States, offering clinicians an additional layer of support in detecting congenital abnormalities. As AI technologies continue to expand in healthcare, prenatal care is emerging as a key area where machine learning can complement human expertise, improving outcomes for both mothers and babies.
Health
Early Flu Wave Hits Europe as Experts Offer Tips to Ease Symptoms

Most people catch a cold in winter, battling fatigue, runny noses, sneezes, coughs, and congestion. This season, an unusually early flu wave has affected millions across Europe, prompting health authorities to offer guidance on managing symptoms.
Typically, influenza season runs from mid-November to late May. However, figures from the European Centre for Disease Prevention and Control (ECDC) indicate that cases in 2025 surged three to four weeks earlier than in the previous two seasons. The agency reports that flu circulation remains high, though it has recently peaked in most regions.
While there are no specific cures for the flu and the common cold, some simple measures can help manage symptoms and improve comfort. Experts recommend a combination of hydration, rest, and symptom relief to support recovery.
Drinking enough fluids is particularly important. Water, broths, and warm beverages with honey, lemon, or ginger can help prevent dehydration, loosen mucus, and soothe irritated airways. Health authorities caution against alcohol, coffee, and other caffeinated drinks, which can act as diuretics and worsen congestion and sore throats.
Gargling with warm salt water is another simple measure to ease throat pain and reduce inflammation. Mixing one teaspoon of salt in a cup of warm water and using it several times a day can provide relief and help clear mucus.
Rest is essential for recovery. Adults are advised to sleep seven to nine hours a night, and to take naps as needed to allow the immune system to combat the virus. Light exercise such as short walks or yoga may be suitable for mild symptoms, but physical activity should be avoided if fever is present or chest symptoms worsen, to prevent complications.
Maintaining moisture in the environment can also alleviate symptoms. Dry air worsens congestion and sore throats, while humidified air hydrates airways and helps clear mucus. Higher humidity can also reduce how long some respiratory viruses survive in the air.
Medicines can relieve symptoms but do not cure viral infections. Decongestants, such as saline sprays or drops, can ease nasal congestion. Painkillers, including ibuprofen, may reduce fever and discomfort in adults. Antibiotics are not effective against colds or flu and should be avoided to prevent antibiotic resistance.
Health authorities emphasize that these measures, combined with good hygiene and vaccination where appropriate, are key to limiting the impact of seasonal viruses. The ECDC notes that most healthy adults recover from flu within about a week, while a common cold may last up to two weeks.
With the early flu wave affecting millions across Europe, simple steps such as staying hydrated, resting, and taking appropriate symptom relief can help patients recover safely and reduce strain on healthcare services.
Health
Climate Change Drives Respiratory Illness, Push for Low-Carbon Inhalers Grows
As climate change worsens respiratory diseases, doctors and drugmakers are exploring earlier diagnosis and low-carbon inhalers to cut emissions from care and protect patients.
For millions of people worldwide, climate change is already affecting breathing. Rising air pollution, longer pollen seasons, and smoke from wildfires are contributing to asthma attacks, lung damage, and other chronic respiratory illnesses. At the same time, the health systems treating these conditions are themselves contributing to greenhouse gas emissions.
Experts note that over 90 percent of the global population breathe air with particulate levels above World Health Organization recommendations. Climate extremes and poor air quality are increasing the frequency and severity of respiratory diseases, from exacerbations to disease onset. Therese Laperre, head of the respiratory department at University Hospital Antwerp, said changes in particulate matter can trigger emergency visits for asthma and chronic pulmonary diseases days later. A European Environment Agency study estimated that more than a third of chronic respiratory disease deaths in Europe are linked to environmental factors such as pollution, extreme temperatures, wildfire smoke, and allergenic pollen.
Worldwide, an estimated 400 to 500 million adults live with COPD, and over 250 million have asthma. Health care responses come with their own climate cost. Health Care Without Harm estimates global health services generate about five percent of worldwide greenhouse gas emissions, which could rise to six gigatons annually by 2050 if unchecked, equivalent to more than a billion cars on the road. Hospitals, particularly intensive care units, are among the most carbon-intensive, due to high energy use, equipment, and single-use materials.
Respiratory specialists emphasize that early disease control benefits both patients and the environment. Philippe Tieghem from the French respiratory association Sante Respiratoire said early diagnosis allows for better patient care while reducing emissions.
Inhalers, commonly used to treat asthma and COPD, illustrate the challenge. Traditional pressurised metered-dose inhalers (pMDIs) use hydrofluorocarbon propellants, which contribute 16–17 million tonnes of CO₂-equivalent emissions globally each year. The UK’s National Health Service estimates these inhalers account for around three percent of its carbon footprint.
Pharmaceutical companies are developing greener alternatives. AstraZeneca’s reformulated COPD inhaler uses a new propellant, HFO‑1234ze(E), cutting its global warming potential by roughly 99.9 percent compared with older devices. The company has pledged to reduce its emissions by 98 percent by 2026, starting with inhalers. Pfizer and Johnson & Johnson have committed to net-zero targets by 2040 and 2045, respectively.
Pablo Panella, senior vice-president for respiratory diseases at AstraZeneca, told Euronews Health that early detection, diagnosis, and treatment keep patients controlled in the community, reducing hospital admissions and the high-carbon interventions that come with them. Panella also stressed the importance of regulation that supports innovation, allowing low-carbon technologies to reach patients more quickly.
Better disease control, combined with environmentally conscious products, is creating the concept of the “green patient”: someone whose chronic condition is managed well enough to avoid repeated flare-ups, hospital stays, and carbon-intensive treatments.
-

 Entertainment1 year ago
Entertainment1 year agoMeta Acquires Tilda Swinton VR Doc ‘Impulse: Playing With Reality’
-

 Business2 years ago
Business2 years agoSaudi Arabia’s Model for Sustainable Aviation Practices
-

 Business2 years ago
Business2 years agoRecent Developments in Small Business Taxes
-

 Home Improvement1 year ago
Home Improvement1 year agoEffective Drain Cleaning: A Key to a Healthy Plumbing System
-

 Politics2 years ago
Politics2 years agoWho was Ebrahim Raisi and his status in Iranian Politics?
-

 Business2 years ago
Business2 years agoCarrectly: Revolutionizing Car Care in Chicago
-

 Sports1 year ago
Sports1 year agoKeely Hodgkinson Wins Britain’s First Athletics Gold at Paris Olympics in 800m
-

 Business2 years ago
Business2 years agoSaudi Arabia: Foreign Direct Investment Rises by 5.6% in Q1